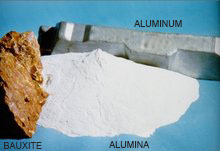
FACT.: It takes 4 tons of bauxite to produce 2 tons of alumina.
The production of the metal Aluminum from the raw ore of Bauxite is a two stage process.
Stage 1
Converting Bauxite to Alumina
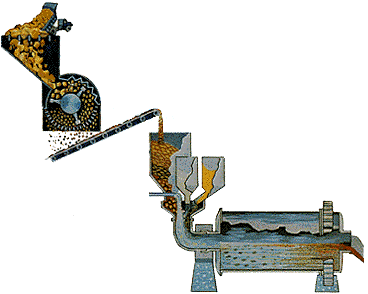
STEP 1 - Crushing and Grinding: Alumina recovery begins by passing the bauxite through screens to sort it by size.
It is then crushed to produce relatively uniformly sized material. This materials is fed into a large grinding mill where it is mixed with a caustic soda solution (sodium hydroxide) under high temperature and pressure.
The grinding mill rotates like a huge drum while steel rods, rolling around loose inside the mill, grind the ore to an even finer consistency. The material finally discharged from the mill is called slurry.
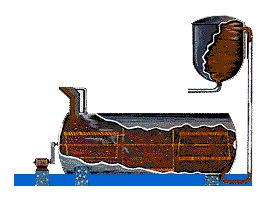
STEP 2 - Digesting: The slurry is pumped to a digester where the chemical reaction to dissolve the alumina takes place. In the digester the slurry - under 50 pounds per square inch pressure - is heated to 300° Fahrenheit (145° Celsius). It remains in the digester under those conditions from 30 minutes to several hours.
More caustic soda is added to dissolve aluminum containing compounds in the slurry. Undesirable compounds either don't dissolve in the caustic soda, or combine with other compounds to create a scale on equipment which must be periodically cleaned. The digestion process produces a sodium aluminate solution. Because all of this takes place in a pressure cooker, the slurry is pumped into a series of flash tanks to reduce the pressure and heat before it is transferred into settling tanks
STEP 3 - Settling: Settling is achieved primarily by using gravity, although some chemicals are added to aid the process. Just as a glass of sugar water with fine sand suspended in it will separate out over time, the impurities in the slurry - things like sand and iron and other trace elements that do not dissolve - will eventually settle to the bottom.
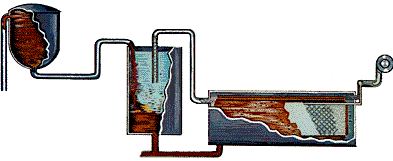
The liquor at the top of the tank (which looks like coffee) is now directed through a series of filters. After washing to recover alumina and caustic soda, the remaining red mud is pumped into large storage ponds where it is dried by evaporation.

The alumina in the still warm liquor consists of tiny, suspended crystals. However there are still some very fine, solid impurities that must be removed. Just as coffee filters keep the grounds out of your cup, the filters here work the same way.
The giant-sized filters consist of a series of "leaves"- big cloth filters over steel frames - and remove much of the remaining solids in the liquor. The material caught by the filters is known as a filter cake and is washed to remove alumina and caustic soda. The filtered liquor - a sodium aluminate solution - is then cooled and pumped to the precipitators.

STEP 4 - Precipitation: Imagine a tank as tall as a six-story building. Now imagine row after row of those tanks called precipitators. The clear sodium aluminate from the settling and filtering operation is pumped into these precipitators.
Fine particles of alumina - called seed crystals (alumina hydrate) - are added to start the precipitation of pure alumina particles as the liquor cools. Alumina crystals begin to grow around the seeds, then settle to the bottom of the tank where they are removed and transferred to "thickening tanks." Finally, it is filtered again then transferred by conveyor to the calcination kilns.

STEP 5 - Calcination: Calcination is a heating process to remove the chemically combined water from the alumina hydrate. That's why, once the hydrated alumina is calcined, it is referred to as anhydrous alumina. Anhydrous means without water.
From precipitation, the hydrate is filtered and washed to rinse away impurities and remove moisture. A continuous conveyor system delivers the hydrate into the calcining kiln. The calcining kiln is brick-lined inside and gas-fired to a temperature of 2,000°F or 1,100°C. It slowly rotates (to make sure the alumina dries evenly) and is mounted on a tilted foundation which allows the alumina to move through it to cooling eqipment. (Newer plants use a method called fluid bed calcining where alumina particles are suspended above a screen by hot air and calcined.)
The result is a white powder like that shown below: pure alumina. The caustic soda is returned to the beginning of the process and used again.
At this point, the alumina is ready for conversion into aluminum at a smelter.
Alumina is also used in making chemicals and ceramics.
Stage 2
Converting Alumina to Aluminum
Smelting: In 1886, two 22-year-old scientists on opposite sides of the Atlantic, Charles Hall of the USA and Paul L.T. Heroult of France, made the same discovery - molten cryolite (a sodium aluminum fluoride mineral) could be used to dissolve alumina and the resulting chemical reaction would produce metallic aluminum. The Hall-Heroult process remains in use today.
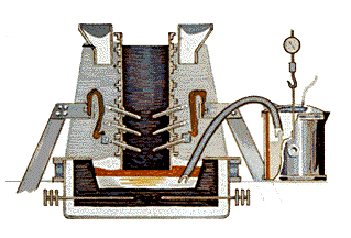
The Hall-Heroult process takes place in a large carbon or graphite lined steel container called a reduction pot. In most plants, the pots are lined up in long rows called potlines.
The key to the chemical reaction necessary to convert the alumina to metallic aluminum is the running of an electrical current through the cryolite/alumina mixture. The process requires the use of direct current (DC) - not the alternating current (AC) used in homes. The immense amounts of power required to produce aluminum is the reason why aluminum plants are almost always located in areas where affordable electrical power is readily available. Some experts maintain that one percent of all the energy used in the United States is used in the making of aluminum.
The electrical voltage used in a typical reduction pot is only 5.25 volts, but the amperage is VERY high - generally in the range of 100,000 to 150,000 amperes or more. The current flows between a carbon anode (positively charged), made of petroleum coke and pitch, and a cathode (negatively charged), formed by the thick carbon or graphite lining of the pot.

When the electric current passes through the mixture, the carbon of the anode combines with the oxygen in the alumina. The chemical reaction produces metallic aluminum and carbon dioxide. The molten aluminum settles to the bottom of the pot where it is periodically syphoned off into crucibles while the carbon dioxide - a gas - escapes. Very little cryolite is lost in the process, and the alumina is constantly replenished from storage containers above the reduction pots.
The metal is now ready to be forged, turned into alloys, or extruded into the shapes and forms necessary to make appliances, electronics, automobiles, airplanes cans and hundreds of other familiar, useful items.
Aluminum is formed at about 900°C, but once formed has a melting point of only 660°C. In some smelters this spare heat is used to melt recycled metal, which is then blended with the new metal.
Recycled metal requires only 5 per cent of the energy required to make new metal. Blending recycled metal with new metal allows considerable energy savings, as well as the efficient use of the extra heat available. The quality of recycled metal is good and great for most all aluminum uses, except in those industries such as aerospace where only very high quality primary aluminum is warranted.
The smelting process required to produce aluminum from the alumina is continuous the potline is usually kept in production 24 hours a day year-round. A smelter cannot easily be stopped and restarted. If production is interrupted by a power supply failure of more than four hours, the metal in the pots will solidify, often requiring an expensive rebuilding process. The cost of building a typical, modern smelter is about $1.6 billion.
Most smelters produce aluminum that is 99.7% pure - acceptable for most applications. However, super pure aluminum (99.99%) is required for some special applications, typically those where high ductility or conductivity is required. It should be noted that what may appear to be marginal differences in the purities of smelter grade aluminum and super purity aluminum can result in significant changes in the properties of the metal.
FACT.:
About 6.2 kWH (kilowatt hours) of electricity is required to
produce one pound of aluminum from alumina.
produce one pound of aluminum from alumina.
Drawings and information courtesy of Reynolds Aluminum,
Alcoa Aluminum and the Aluminum Institute.
Alcoa Aluminum and the Aluminum Institute.